Biosafety and Biosecurity Policy
Optimizing Security of Biological Research
United States federally funded research must benefit American citizens without jeopardizing our Nation’s security, strength, or prosperity. This requires balancing the prevention of catastrophic consequences with maintaining readiness against biological threats and driving global leadership in biotechnology, biological countermeasures, biosecurity, and health research.
- Executive Order on Improving the Safety and Security of Biological Research
- Terminating or Suspending Dangerous Gain-of-Function Research in Accordance with the Executive Order on Improving the Safety and Security of Biological Research
- Implementation Update: Improving the Safety and Security of Biological Research
External Resources
For information on policies related to dual use research of concern and enhanced potential pandemic pathogens please see:
https://aspr.hhs.gov/S3/Pages/High-Consequence-Research-Oversight.aspx
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) detail safety practices and containment procedures for basic and clinical research involving recombinant or synthetic nucleic acid molecules, including the creation and use of organisms and viruses containing recombinant or synthetic nucleic acid molecules.
All IBC minutes resulting from meeting taking place on or after June 1, 2025, must be posted on a public-facing institutional website. For more information, please see the guidance below. Updated FAQs on IBC meetings and minutes are available along with a new resource that details Points to Consider when generating IBC meeting minutes and includes an IBC Minutes Template to assist IBCs in producing meeting minutes that adequately document fulfillment of their biosafety review and oversight responsibilities:
Incident Reporting
The NIH Guidelines require that any significant problems, violations, or any significant research-related accidents and illnesses” be reported to OSP within 30 days. Appendix G of the NIH Guidelines specifies certain types of accidents that must be reported on a more expedited basis. Specifically, Appendix G-II-B-2-k requires that spills and accidents in BL2 laboratories resulting in an overt exposure must be immediately reported to the OSP (as well as the IBC). In addition, Appendices G-II-C-2-q and G-II-D-2-k require that spills or accidents occurring in high containment (BL3 or BL4) laboratories resulting in an overt or potential exposure must be immediately reported to OSP (as well as the IBC and BSO).
*Incident reports may be released to the public in full. Please note that incident reports should not include personally identifiable information or any information that you do not wish to make public. Proprietary, classified, confidential, or sensitive information should not be included in the report. If it is necessary to include such information, please clearly mark it as such so that it can be considered for redaction in accordance with Freedom of Information Act exemptions.*
IBC RMS and Registration Information
IBC Self-Assessment Tool
Investigator Brochure
Modernizing and Strengthening Oversight of Biosafety
NIH is launching a new Biosafety Modernization Initiative to strengthen biosafety policies, practices, and oversight to keep pace with the evolving risks posed by today’s rapidly advancing science and technology. As part of this initiative, NIH will modernize our existing biosafety policy to holistically address the emergent biosafety needs of today.
NIH will also strengthen our partnerships with institutional oversight bodies to empower Institutional Biosafety Committees, reinforcing their position as the front line of local oversight, akin to other oversight structures like Institutional Review Boards and Institutional Animal Care and Use Committees required for ensuring a safe, responsible research ecosystem.
The goal is to fulfill NIH’s commitment to ensuring that gold standard science is conducted under gold standard biosafety conditions, ushering in a more effective, transparent, and modern biosafety system.
Timeline
The Biosafety Modernization Initiative will unfold over the next year, with regular opportunities for the public and expert engagement nationwide. For this initiative, we will prioritize:
- Transparency in how we communicate and carry out our work
- Open and inclusive dialogue, including a willingness to challenge the status quo
- Multiple methods of engagement, actively seeking input from both experts and the public
We’re also taking a phased approach – see below for key milestones and what to expect at each stage.
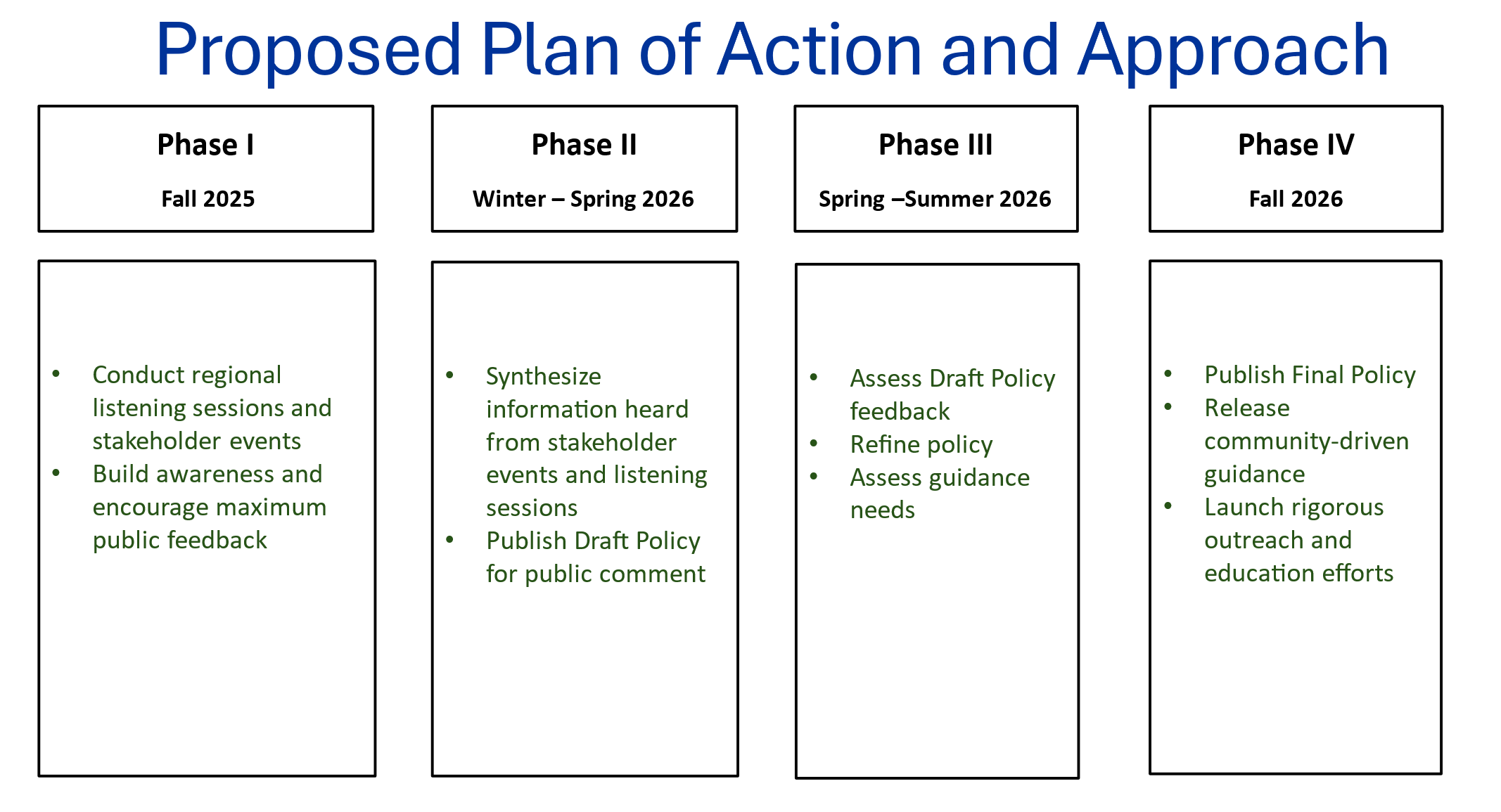
Engagement Opportunities
NIH will engage early and often, implementing a comprehensive outreach and engagement strategy to ensure that 21st century biosafety oversight meets the research needs of both today and tomorrow. To support this effort, NIH will initially host focus groups across the country to discuss the shift from a technique-based to a risk-based biosafety framework.
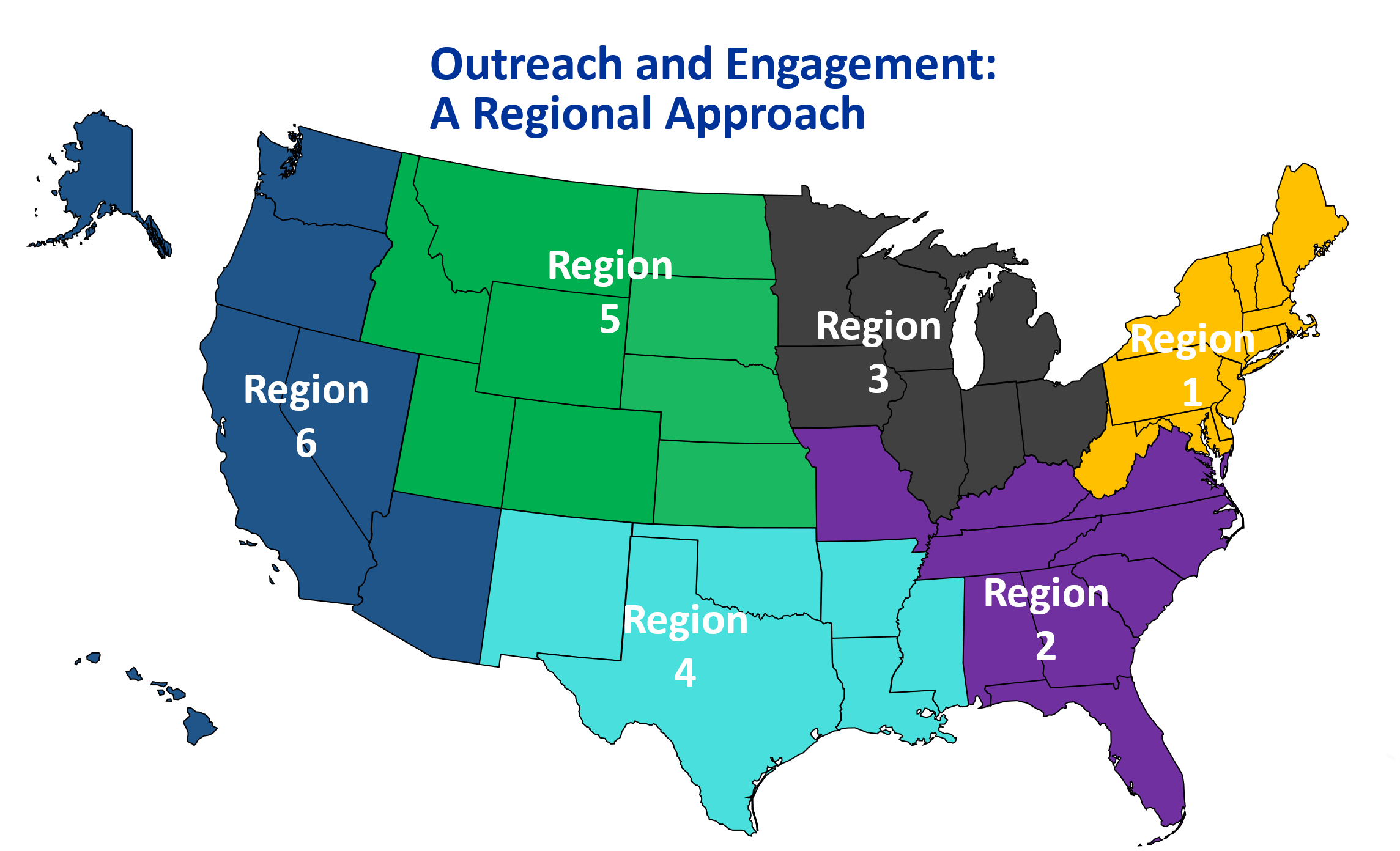
Engagements:
- Region 1 Listening Session – September 30, 2025
- Region 2 Listening Session – December 17, 2025
- Region 3 Listening Session – January 15, 2026
- Region 4 Listening Session – January 29, 2026
- Region 5 Listening Session – February 12, 2026
- Region 6 Listening Session – February 26, 2026
Share Your Thoughts with On-Demand Commenting
NIH wants to hear from all stakeholders on their ideas for how to strengthen and modernize biosafety. At any time, take advantage of on-demand commenting by visiting: https://osp.od.nih.gov/help-modernize-and-strengthen-the-oversight-of-biosafety/.
On this page, you will find information on how to provide real-time feedback and as well as how you can help spread the word about NIH’s biosafety efforts.
Contact Us
We encourage our stakeholders to share their feedback on biosafety modernization at any time. To get in touch, please email us at [email protected].
Links to Other Biosafety Resources
Additional Resources
- CDC Biosafety Resources and Tools
- American Biological Safety Association (ABSA)
- AIHA Home Page
- American Society for Microbiology
- The American Society of Gene and Cell Therapy
- Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) Website
- The Centers for Disease Control and Prevention (CDC) Website
- The US Department of Health and Human Services (HHS)
- The Office for Human Research Protections (OHRP)
- The Federal Register Website
- The Office of Laboratory Animal Welfare
- The Animal and Plant Health Inspection Service (APHIS) Website
- Biosafety in Microbiological and Biomedical Laboratories (BMBL)
- Risk Group Classification for Infectious Agents (ABSA)
- Select Agent Program
- Association for the Accreditation of Human Research Protection Programs
- Biosafety Discussion List

